
Electronic english version since 2022 |
The newspaper was founded in November 1957
| |
|
Number 33 (4731) |
About what the newspaper wrote on this day 60 years ago
There is element 104!
№70 (130) 29 August, 1964
As reported to the TASS correspondent by the State Committee for the Use of Atomic Energy of the USSR, experiments on the synthesis of transuranic elements were carried out for a number of years at the Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research (Dubna) under the supervision of Corresponding Member of the USSR Academy of Sciences G.N.Flerov.
These experiments were crowned with a new success - the discovery of a short-lived radioactive emitter. This emitter has a lifetime of 0.3 seconds relative to spontaneous decay and is synthesized at a rate of one atom every 5 hours when plutonium-242 is bombarded with accelerated ions of neon-22. More than 150 new nuclei have been synthesized.
At present, the analysis of the data obtained and the implemented diverse control experiments result with high reliability in the conclusion that the new emitter is an isotope with an atomic weight of 260 of the previously unknown element 104 of the Periodic Table of Mendeleev. Preliminary data on these results were reported in July of this year at the Congress on Nuclear Physics in Paris.
Experiments on further investigations of the physical and chemical properties of the new element are currently underway.
(TASS)
Beyond the edge of the Periodic Table
 |
| Georgy Flerov |
According to current ideas, the element 104 should open a completely new family of the heaviest elements in the Periodic Table. As it is known, the element 103 - lawrencium became the "closing" element of the heaviest group of actinides that opens with number 90 - thorium and after number 92 - uranium, all of them obtained artificially in laboratories.
What is next, what is hidden beyond the edge of the Table? What amazing secrets of nature can the element 104 reveal? What properties will it have?
Attempts to answer these questions, to synthesize and isolate element 104 with sufficient reliability have long been underway at the University of California in Berkeley under the supervision of Professor Albert Ghiorso and at the Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna under the supervision of Corresponding Member of the USSR Academy of Sciences Georgy Flerov.
But with each subsequent cell of the Periodic Table beyond uranium, the difficulties increase. To put it modestly, in geometric progression. Nature, as if trying to hold on to its secrets, retreats more and more reluctantly, becoming more and more stubborn and cunning.
If the first transuranium element - plutonium was "discovered" in a quantity that fit on the tip of a needle, then artificially synthesized heavier elements - californium, berkelium, einsteinium were at first completely impossible to see with the naked eye. The element 101 - mendelevium was "mined" with incredible difficulty in the amount of 17 atoms and the element 103 - lawrencium was obtained so much that there could be no talk of isolating and determining it chemically, although chemists working in this field, as they say, surpassed themselves and learned to handle practically unobservable weight quantities of substances. Still, we had to limit ourselves to just physical evidence of the discovery of the new element.
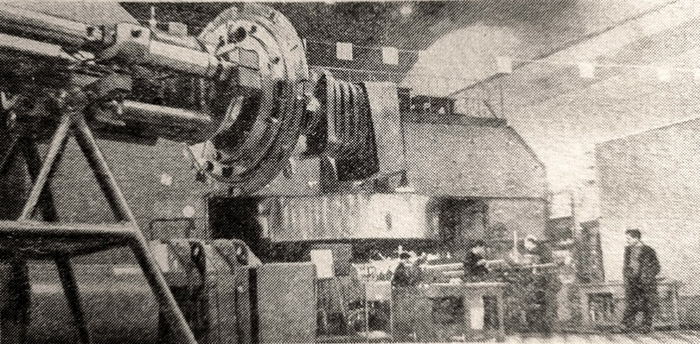
Cyclic accelerator of multi-charged ions
But the difficulties not only did not stop, yet, it seemed, even more spurred on the physicists. In addition to their invaluable value for science, transuranics have already done a good service to technology. Plutonium, for example, can now be produced on an industrial scale and it turned out to be a valuable nuclear fuel, allowing for the generation of economically viable electric power. Curium may well become an excellent compact source of heat. Californium and heavier elements - compact sources of neutrons and who knows what other properties these heaviest "bricks" of the Universe conceal!
Nature hid the element 104 with particular care. Therefore, both American and Soviet physicists developed experimental equipment of increased precision and selectivity.
The most convenient and already tested way for synthesizing other heavy saurans is the reaction of interaction of charged particles accelerated in cyclotrons or linear accelerators with nuclei of a specially selected target substance. In order for the desired reaction to occur, the staggered and accelerated charged particle should penetrate the nucleus to the target substance and unite with it. By selecting the particles and material for the target, one can hope to achieve the desired result.
To obtain element 104, the ideal case would probably be to use the heaviest of the known elements, lawrencium, as a target and the smallest of the charged particles, the hydrogen nucleus, the proton, as a projectile (the sum of the atomic numbers should result in 104). However, lawrencium does not yet occur even in submicroscopic quantities and its use today is an unattainable dream. Therefore, American physicists, for example, bombarded a californium target (atomic number 98) with accelerated carbon ions (atomic number 6). So far, the desired effect has not been observed. Soviet physicists that have the most powerful cyclic accelerator of multiply charged ions in the world, decided to use "heavy artillery". A target of plutonium-242 (atomic number 94) was bombarded with multiply charged ions of heavy neon-22 (atomic number 10).
In theory, a plutonium nucleus, having captured a neon ion, should enter an excited state and having evaporated four neutrons, turn into a heavy isotope of element 104 with a mass number (the total number of protons and neutrons in the nucleus) of 260. However, the probability that a nucleus from a target, having captured a charged particle, will not instantly fly apart is very small. The higher the atomic number, the smaller and smaller it becomes. Thus, during the synthesis of the heavy isotope of element 102, only one in a hundred million of all interactions was realized as required. It was assumed that 104 could be produced in only one in ten billion of all possible cases. Theorists predicted its lifetime to be something around a millionth of a second. But according to estimates based on experimental data, contrary to the opinion of theorists, it turned out that element 101 should decay within one thousandth of a second to a second. This is also a very short time available for detection.
Armed with such meager and uninspiring information, a group of young physicists, chemists and mechanics from the Laboratory of Nuclear Reactions - Yuri Oganessian, Yuri Lobanov, Vladislav Kuznetsov, Viktor Druin, Vladimir Perelygin, Krasnoslav Gavrilov, Svetlana Tretyakova, Vasily Plotko, under the supervision of Corresponding Member of the USSR Academy of Sciences Georgy Flerov, started producing the most interesting and mysterious element that does not exist in nature, for which it is not even known where to prepare a place in the Periodic Table - its properties can bring many surprises not only to physicists, but also to chemists.
But because of such vague information and unclear estimates, there was naturally a danger of mistaking any previously unknown emitter that appeared in reactions for the element 104. And indeed, in the very first experiments, nuclei were discovered that decayed in 13 thousandths of a second. So, I wanted to believe that this was the long-awaited 104! But the law of the Laboratory of Nuclear Reactions consists of multiple rechecks and strict requirements for irrefutable evidence. Therefore, to the great chagrin of the researchers, the control experiments they themselves had carried out showed that this was not the 104, but as it turned out later, an isomer of americium-242 (atomic number 95), for some reason with a greatly facilitated ability to fission. In further experiments, many similar emitters were found with a lifetime of one thousandth of a second, 3 seconds and even 200 seconds. But none of them were the 104. Thousands of nuclei of the heavy isotope 256 of element 102 were added to them, since not only neon (10) but also its "fragments" - oxygen (8), carbon (6), beryllium (4) interacted with the target plutonium.
All this complicated the matter even more. The extremely strong background of decaying isomers and nuclei of the element 102 made the task almost hopeless. In the general mess of various nuclei, it seemed impossible to find and recognize several barely distinguishable grains even with the help of very sensitive equipment.
Whole series of new techniques were developed that made it possible to significantly reduce the background and gave hope of catching nuclei of the 104 in narrow, about tenths of a second, "gaps" in time between other nuclei.
A probe was made - a device that included a target and detectors (detecting agents) that could be positioned under a beam of charged particles of a cyclotron. In parallel, another - a tape probe was produced. If in the first, nuclei knocked out of the target were transferred to the detector-registrators by a rotating disk, then in the second, they were picked up by a continuously moving endless nickel tape. Along the tape were specially made glasses that preserved the imprint of only the nuclear fragments and did not react to all other types of radiation. The tape speed, like the disk speed, could be changed. Thus, like in a movie with slow motion, the fissioning nuclei were stretched and clearly separated in time and it was easier to "sort" them.
The researchers did not leave the cyclotron for days, selecting its full power. For reliability, more accurate control and a guarantee in case of breakdown, the experiments were carried out on both probes. They changed the speed of the tape and disks, the distances between the glasses.

From left to right: mechanic V.Plotko, physicists Yu.Oganessian and V.Druin are preparing the disk probe for the experiment
...It was titanic work, compared to which manual extraction of ore from the mines seems like child's play. Drouin and Oganessian counted the time spent at the machines not in days, but in months.
Finally, the devices registered an emitter with a lifetime of three-tenths of a second. The result was so timid, barely perceptible, like a splash, that the experimenters themselves with bitter irony called it a "wart" on the decay curve. Despite it, the experimenters managed to study the dependence of the element's yield on the energy of the incident ions. It turned out that this dependence corresponds to the supposed evaporation of four neutrons from the compound nucleus.
Again, the tapes and glasses were changed, again there was a struggle for atoms. It was necessary to at least slightly increase the sensitivity of the equipment. Krasnoslav Gavrilov was looking for more and more optimal techniques of preparing targets.
And when bombarding plutonium-242 targets with neon-22 ions, an emitter with a lifetime of 0.3 seconds was clearly, reliably and stably registered. Everything indicated that this was a heavy isotope of element 104 with a mass number of 260.
But, as usual, attempts to disprove the obvious began again in the laboratory. To ensure against a possible error, uranium-238 was irradiated with neon-22 and plutonium-242 with oxygen-18 and neon-20 ions. In all these reactions, the element 104 with a mass of 260 cannot be produced. And indeed, no emitter with a lifetime of 0.3 seconds was detected.
The main and control experiments show with great certainty that the element 104 with a mass number of 260 has been obtained. More than 150 atoms of the new, hitherto unknown element have already been obtained. How much is this? One can judge by the fact that in 5-6 hours only one atom of the 104 is produced, while mendelevium, first obtained in the amount of 17 atoms, had a yield of 1 atom per hour. Element 104 that is to become the head of a whole, as yet unknown family of the Periodic Table is studied in detail. Physicists reveal its character and habits. Czechoslovakian and Soviet chemists have prepared a unique technique for studying its chemical properties.
The "newborn" has not yet received a name. But this task is not as difficult as its discovery. To do it, you will not have to leaf through tables and reference books for a long time, finding out whose contribution to nuclear physics was the most significant. Nuclear physics knows the names of scientists whose enormous scientific contribution united selfless work for the benefit of humanity.
E.Knorre, scientific observer of the Academy of Pedagogical Sciences
***
A press conference was held at the Laboratory of Nuclear Reactions on 27 August. It was opened by JINR Director Professor D.I.Blokhintsev. He congratulated the laboratory staff on the great victory and wished them further success. Afterwards, Director of the Laboratory of Nuclear Reactions Professor G.N.Flerov and Head of the scientific group of chemists I.Zvara spoke.
The Press Conference was attended by Vice-Director Professor I.Ulegla, Vice-Director E.Fenvesh and the scientific group that made the discovery of the element 104.
Columnist Irina LEONOVICH,
photo by Yury TUMANOV